The Role of Oncogene Activated ER Stress in Skin and Lung Tumor Development
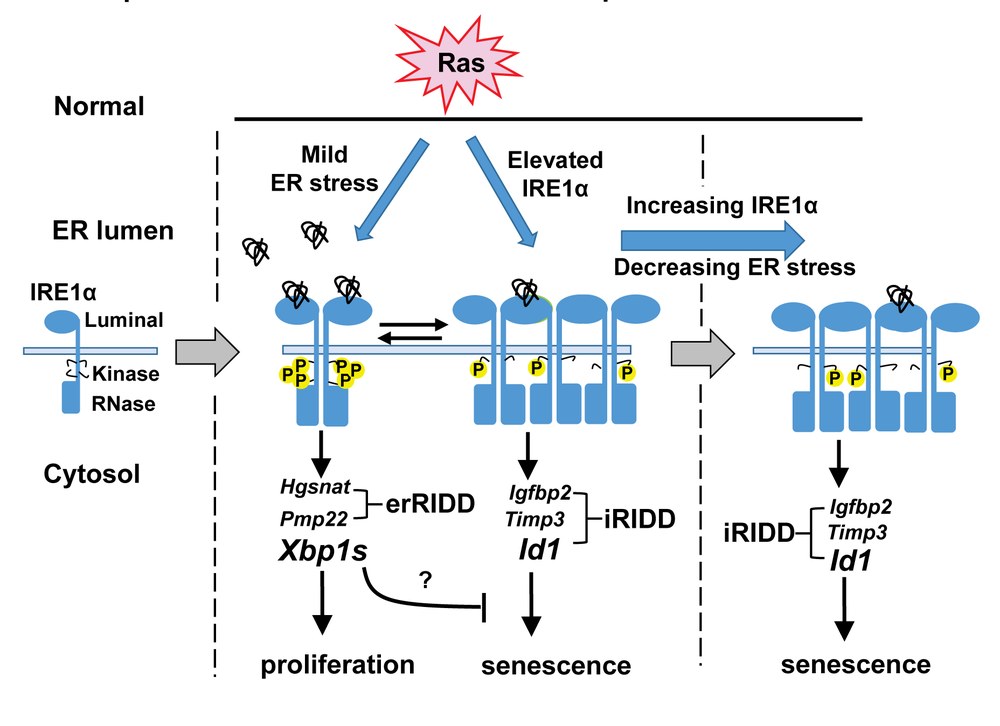
The adaptive response of cells to increased unfolded proteins within the endoplasmic reticulum, or the unfolded protein response (UPR), is a fundamental and conserved cellular survival mechanism. Cancer development is often associated with a range of cytotoxic conditions like hypoxia, nutrient deprivation, and pH changes. These conditions trigger a set of cellular stress response pathways including the ER-stress response. Many aspects of the endoplasmic reticulum (ER)-stress response are adaptive and protect tumor cells from cell death suggesting a crucial role in tumor growth. Much less is known about the role of ER-stress response pathways in early stages of cancer and tumor progression. IRE1α is a major transducer of the UPR and is an ER localized transmembrane protein kinase/RNase. When activated by ER stress IRE1α splices an internal sequence from the XBP1 mRNA to generate a new transcript encoding the active XBP1 transcription factor, a major regulator of the adaptive response to ER stress. In addition the IRE1α RNase can degrade a number of mRNAs in a process called Regulated IRE1α Dependent Decay (RIDD). This function of IRE1α reduces levels of mRNA's that encode proteins processed through the ER but also mediates cell death to in response to unremitting ER stress. Both IRE1α and spliced Xbp1 are elevated in cancer, and specific somatic mutations within the IRE1α kinase and RNase domains have been identified in cancers but the function consequences of these are unknown.
We are currently studying Ras oncogene activation of the IRE1α pathway in primary epidermal keratinocytes, and in mouse and human tumor cell lines. The goals of this project are as follows:
- Determine mechanism of IRE1α activation and upregulation by oncogenic Ras, and the interaction between Ras signaling and ER stress in the specific output of the IRE1α RNase.
- Determine if Xbp1 splicing and RIDD functions of IRE1α RNase have opposing roles in Ras-driven cancer.
- Determine the effect of human IRE1α cancer mutants on Ras induced senescence and tumor progression.
This project is supported by the grant 1R01CA197942 to ABG

