Our laboratory is focused on the molecular biology of mammalian biotransformation.
Biotransformation refers to the process by which foreign and endogenous chemicals are metabolized. Human variability in the biotransformation function can substantially impact individual responsiveness to pharmaceuticals and to toxicant exposures. The cytochrome P450s are key enzyme components of the drug and chemical metabolism pathway.
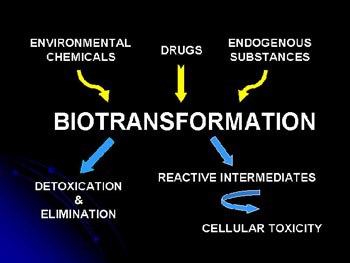
One line of laboratory endeavor relates to the study of the regulatory mechanisms responsible for controlling the expression of the biotransformation enzymes. Recently, a variety of ligand-activated nuclear receptors have been characterized that serve as "xenosensors" in mammalian cells and function as transcriptional activators of a variety of genes, including the cytochrome P450s. Phenobarbital (PB) is an example of clinical compound that produces marked activation of the expression of several P450 genes; a process now known to be regulated by the constitutive androstane receptor (CAR). Our laboratory studies signaling cascades activated by inducer exposures and nuclear events dictating altered DNA-protein and protein-protein interactions associated with the induction process. Experimental models in use include primary hepatocyte cultures, transgenic mouse systems and DNA microarrays. Investigations are underway examining the transcriptional activation processes involved in gene induction, such as the interactions of nuclear receptors with core DNA enhancer elements and the associated network of nuclear co-activator and co-repressor proteins. Recently, we have characterized a series of splice variants for human CAR that lead to structural alterations in receptor function. We are very interested in determining genetic variation in receptor structure that may impact important clinical and toxicological outcomes. These areas of research fall under the heading of "toxicogenomics."
Parallel studies in our laboratory involve characterization of the human epoxide hydrolases, including their structure, regulation and genetic variability. These enzymes, like the cytochrome P450s, function to biotransform a variety of drug intermediates and toxic foreign substances, and may act upon the epoxide intermediates generated by P450 oxygenations. Epoxide hydrolase activity often renders the xenobiotic epoxides less chemically reactive, although bioactivated products may also be produced in certain cases. Our research has focused on two forms of epoxide hydrolase, encoded by the microsomal (EPHX1) and the soluble (EPHX2) genes. The microsomal enzyme is active against a broad array of xenobiotic chemicals, including the cancer-causing polyaromatic hydrocarbons found in smoke. The soluble enzyme participates in the metabolism of endogenous substances  such as the epoxyeicosotrienoic acids, arachidonic acid-derived epoxides. These latter compounds play key roles in processes such as blood pressure control and inflammation. Our epoxide hydrolase research program involves analysis of genetic variation in human populations, structure-function relationships, and the tissue-specific regulation of gene expression. In these investigations, our laboratory team at Penn State works closely with several interdisciplinary centers, including the Huck Institutes of the Life Sciences, the Penn State Institutes of the Environment, and the Fred Hutchinson-University of Washington Consortium in Toxicogenomics. Our research is supported by grants from the National Institute of General Medical Sciences and the National Institute of Environmental Sciences.
such as the epoxyeicosotrienoic acids, arachidonic acid-derived epoxides. These latter compounds play key roles in processes such as blood pressure control and inflammation. Our epoxide hydrolase research program involves analysis of genetic variation in human populations, structure-function relationships, and the tissue-specific regulation of gene expression. In these investigations, our laboratory team at Penn State works closely with several interdisciplinary centers, including the Huck Institutes of the Life Sciences, the Penn State Institutes of the Environment, and the Fred Hutchinson-University of Washington Consortium in Toxicogenomics. Our research is supported by grants from the National Institute of General Medical Sciences and the National Institute of Environmental Sciences.

